More than one hundred women are filing a lawsuit against a pharmaceutical company saying they became pregnant after taking birth control pills that were incorrectly packaged.
The Philadelphia Inquirer reports the case involves Qualitest Inc. which is headquartered in Malvern, Chester County.
The suit also names Vintage Pharmaceuticals, LLC, Endo Health Solutions, Inc., and Patheon Inc., companies that either made or packaged the recalled contraceptives.
The lawsuit seeks money for the costs of delivering and raising the children born as a result of unplanned pregnancies caused by the packaging error.
The Food and Drug Administraion recalled the pills in 2011.
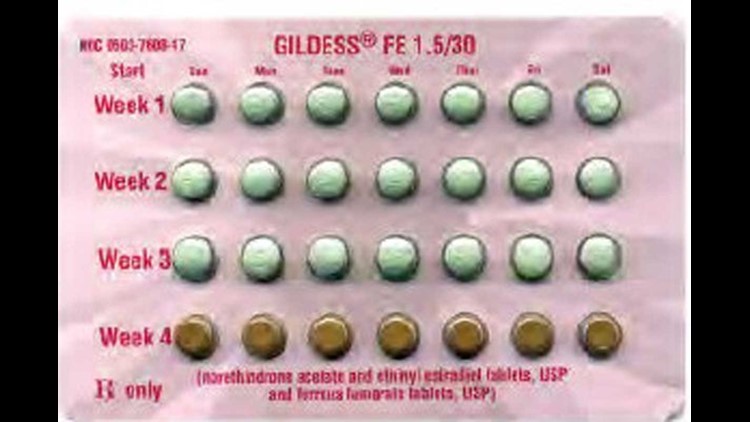
The FDA recall was triggered when a Kansas City woman returned a package to her pharmacist after noticing the blister pack had been rotated 180 degrees reversing the weekly tablet orientation, according to the suit filed in Philadelphia Court of Common Pleas.
When turned around, the placebo or “reminder” pills would be taken the week following a women’s menstrual cycle, greatly increasing her chance of pregnancy.
A federal judge in Georgia rejected the request for class action status for potential victims on November 4th, before lawyers refiled in Pennsylvania.
According to the court order issued in Georgia on Nov. 4, lead attorney Keith Bodoh represents 117 women from 26 states. Of Bodoh’s clients, 113 became pregnant and 94 carried their babies to term.
The birth control trademarks named in the suit include Cyclafem 1/35, Cyclafem 7/7/7, Emoquette, Gildess FE 1.5/30, Gildess FE 1/20, Orsythia, Previfem, and Tri-Previfem. There is no evidence that the problem has recurred.