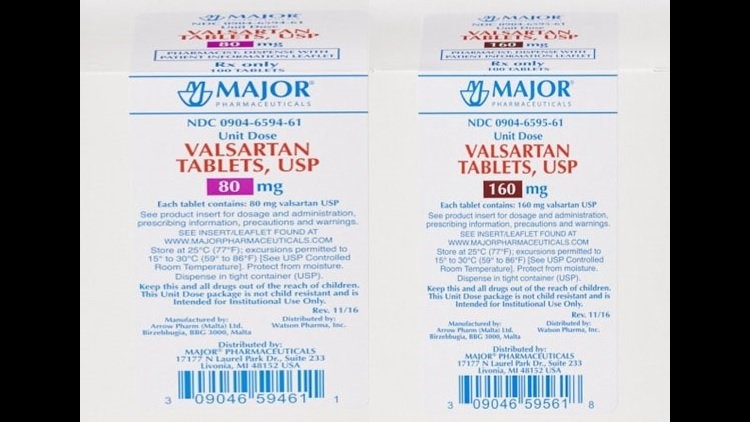
The US Food and Drug Administration said it found an additional “unexpected impurity” in three lots of Torrent Pharmaceuticals’ recalled valsartan drug.
Several pills that contain valsartan, a generic ingredient that helps people with high blood pressure and heart failure, have been under a voluntary recall since July. The drugs were tainted with N-nitrosodimethylamine, or NDMA, an impurity that is considered a possible carcinogen by the US Environmental Protection Agency. It’s an organic chemical used to make liquid rocket fuel and a byproduct from manufacturing some pesticides and processing fish. NDMA can be unintentionally introduced into manufacturing through certain chemical reactions.
Not all versions of the drugs have been recalled, but the FDA keeps a regularly updated list of the drugs that have been impacted.
On Thursday, the FDA said that three lots of the drugs made by Torrent Pharmaceuticals were contaminated with a second impurity, N-Nitrosodiethylamine, or NDEA, which is also a suspected human carcinogen. The agency began testing the recalled products and the pills that have not been recalled for the substance after it learned that Zhejiang Huahai Pharmaceuticals found NDEA in several batches of its valsartan active ingredient. Not all batches have been found to be contaminated.
In response to the second impurity being identified, Health Canada also released guidance on what patients taking affected valsartan medications should do.
What to do if you take valsartan
If you are worried your drug could be on the recall list, talk with your doctor or pharmacist before changing any routine with your medicine. Because not all valsartan drugs are involved in the recall, they might be able to switch you to a version of the drug made by another company.
If you know your drug is on the recall list, the FDA suggests you continue taking it until your doctor or pharmacist provides a replacement.
The FDA said it will continue to test all products containing valsartan for NDEA impurities as well as the NDMA. Testing should wrap up in the next few days.
“As we continue to investigate the root cause of the impurities found in products that contain valsartan, our scientists are testing these products to better understand these impurities and to ensure they’re not present in other products. We’re also taking steps to make sure we’re providing stringent oversight of manufacturing processes to reduce the likelihood that impurities could be introduced into other products,” said FDA Commissioner Dr. Scott Gottlieb. “As we expand our investigational efforts, we’ll continue to make sure the public has the most up-to-date information. We’ll also continue to work with global regulatory agencies to learn as much as we can about how these impurities came about and how they may affect patients’ health around the globe.”