YORK, Pa. — According to the U.S. Food and Drug Administration, 6.5 million Americans suffer from Alzheimer's disease.
A newly approved FDA drug is meant to prevent and stop progression in those who have Alzheimer's disease. Research shows that 20% of African Americans make up that number of people who will suffer from the disease.
Researchers at the Alzheimer’s Clinical Trial Consortium are trying to get ahead of the disease by asking African Americans to take part in clinical trials.
Stephanie Monroe is a member of the Alzheimer’s Clinical Consortium and director of Diversity, Equity, Inclusion and Access at USAgainstAlzheimers. Monroe said since African Americans are more likely to be diagnosed with Alzheimer's disease, her organization wants to specifically know if the drug is safe in Black communities.
“80% of people tell us no one has even talked to them about their brain health and 80% of people tell us no one has asked them to participate in research,” said Monroe. “The same percent tell us if they were asked they would do it, so it’s not true that Blacks won’t get involved. We’ll get involved if we’re asked.”
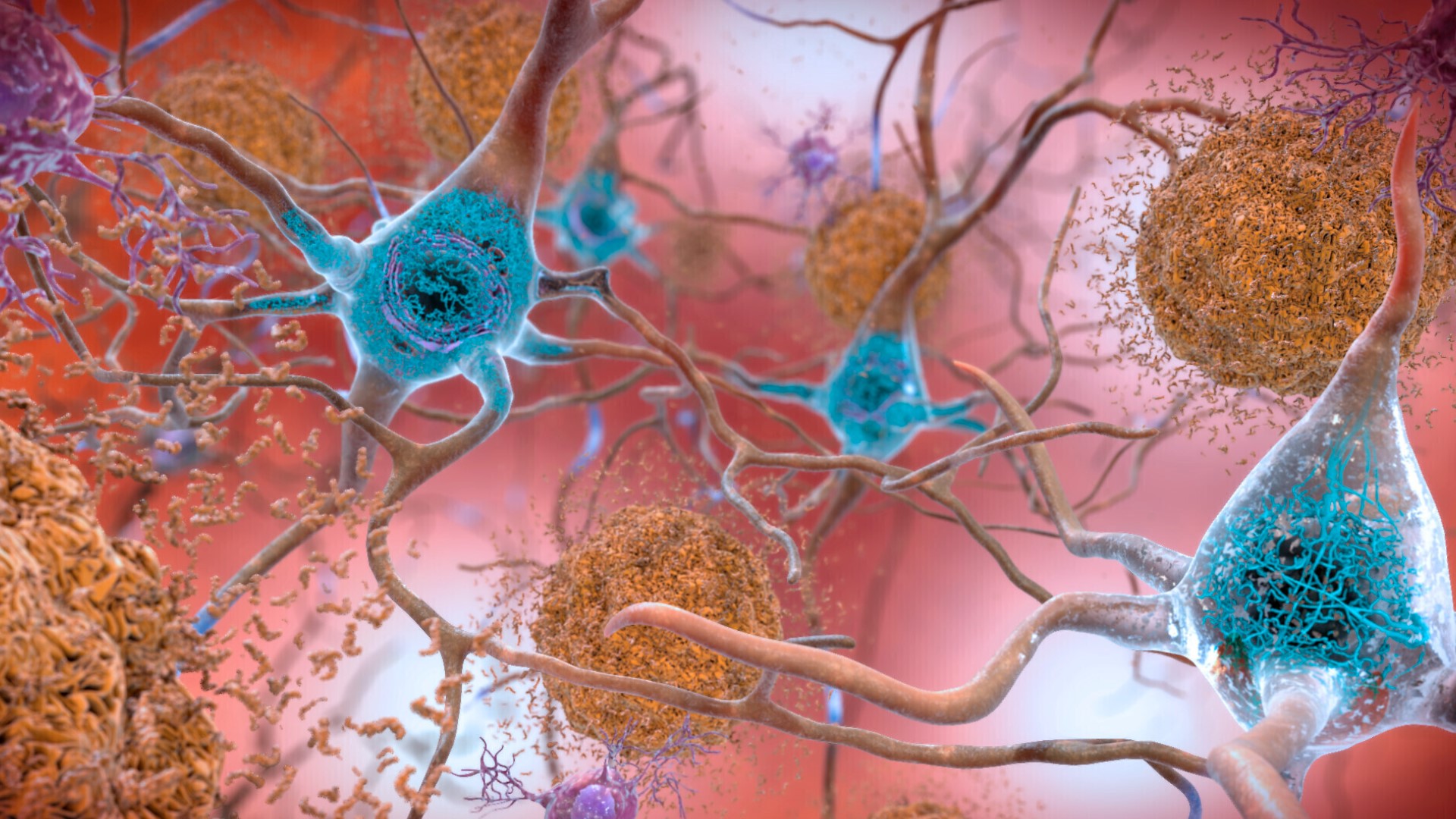
The new FDA-approved drug, called Leqembi, was administered to patients with amyloid beta pathology, which is the hallmark feature of Alzheimer’s disease. Monroe says the drug will prevent a patient from developing Alzheimer’s disease, stop the progression in its tracks or delay symptoms.
“We need to really understand by testing all populations if that goal is going to be realized, or if we are delaying symptoms which are all great things… we haven’t had the ability to do any of that, so we’re really trying to prevent the disease in terms of symptoms or stop the disease in its tracks,” said Monroe.
Participants have to be between the age of 55 and 80 to take part in the trial. To learn more about the clinical trial or get involved, click here.