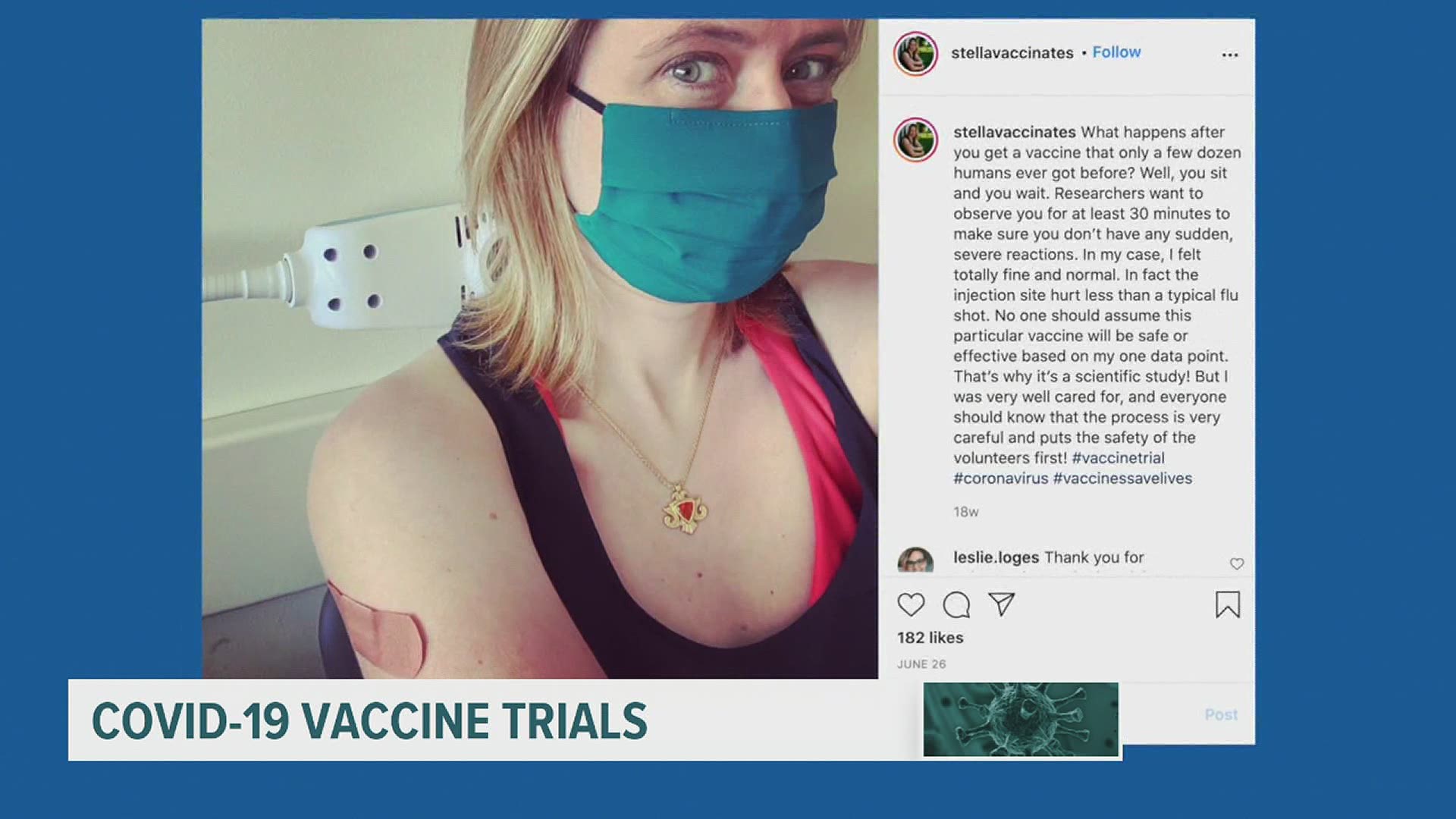
LANCASTER, Pa. — A Lancaster mother is doing her part amid the COVID-19 pandemic by participating in a clinical trial for a COVID-19 vaccine. Stella Sexton, 41, signed up for the INOVIO COVID-19 Vaccine Phase 1 Clinical Trial by Penn Medicine and the Perelman School of Medicine.
Sexton said she stumbled up the trial’s need for volunteers on the Lancaster County’s website at the height of the coronavirus pandemic.
“This was back in May and of course I had been home with my kids and trying to get them through school,” Sexton explained. “I was shopping for my elderly relatives and I was trying to keep everybody going. I just thought ‘Gosh, we need to get a vaccine to end this.’”
The healthy mother of two felt she had a chance to make a difference. She signed up for the DNA vaccine trial and received her first dose on June 8. Sexton received a booster shot one month later.
“The interesting thing about this vaccine is that they have basically stripped those little spikes off the [virus] so it doesn’t contain the whole virus. It can’t make me contagious and it can’t make me sick,” Sexton added. “The goal is to see what my immune system does just with the vaccine.”
After injecting the vaccine, nurses use a device that delivers electrical pulses to your skin, said Sexton, to open up the pores of your skin cells and allow the DNA to get into your skin.
The global coalition of collaborators and partners assembled by Inovio includes the scientific team at the Wistar Institute, whose contributions included key research, and the University of Pennsylvania, who is conducting the clinical trial. Inovio Pharmaceuticals received a $5 million grant from the Bill & Melinda Gates Foundation to accelerate the testing of the CELLECTRA 3PSP device to deliver the vaccine.
The DNA vaccine trial lasts 52 weeks. Sexton has routine bloodwork done and has been documenting the process on her Instagram page, @stellavaccinates. She is sharing her journey with the world to educate people who may be curious about a vaccine.
“I know it seems fast and that they are rushing it, but I think people should understand that ever since SARS-CoV-1 there was all this research that happened into coronavirus vaccines. That research is what we’re benefitting from now,” Sexton said.
The hope is that future generations will benefit from the research scientists are learning today—all because of people like Sexton.
“To me, it felt like something I had to do for my family, something I had to do for my grandparents and my older relatives, but also I really feel that it’s a patriotic duty,” added Sexton.
Other clinical trials for a COVID-19 vaccine are currently enrolling volunteers. If you are interested in participating, visit coronaviruspreventionnetwork.org for more information.